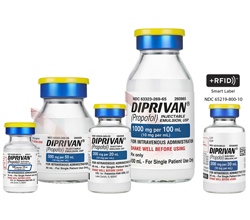
| Unit of Sale NDC |
Unit of Use NDC |
Product Number |
Description |
Strength
(Concentration) |
Fill Volume
(Container Size) |
Closure |
Pack Size |
Min. Order Qty. |
Package Insert |
| 63323-269-50 |
-- |
260950 |
Single-Patient Use Only |
500 mg per 50 mL
(10 mg per mL) |
50 mL
(50 mL) |
32 mm |
20 |
1 x 20 |
|
| 63323-269-65 |
-- |
260965 |
Single-Patient Use Only |
1,000 mg per 100 mL
(10 mg per mL) |
100 mL
(100 mL) |
32 mm |
10 |
1 x 10 |
|
| 63323-269-29 |
63323-269-22 |
260929 |
Single-Patient Use Only |
200 mg per 20 mL
(10 mg per mL) |
20 mL
(20 mL) |
20 mm |
10 |
1 x 10 |
|
| 63323-269-10 |
-- |
260910 |
Single-Patient Use Only |
100 mg per 10 mL
(10 mg per mL) |
10 mL
(10 mL) |
20 mm |
10 |
1 x 10 |
|
| 65219-800-10 |
65219-800-01 |
RF260929 |
Single-Patient Use Only
(RFID Smart Label) |
200 mg per 20 mL
(10 mg per mL) |
20 mL
(20 mL) |
20 mm |
10 |
1 x 10 |
|
-
Contains ethylenediaminetetraacetic acid (EDTA)
Diprivan® is a registered trademark of Fresenius Kabi USA, LLC